July 8, 2019 | Jola Glotzer
Defining the NSD2 interactome
Two CBC Awardees, Jonathan Licht and Neil Kelleher, identify posttranslational modification crosstalk that may play a role in carcinogenesis
Congratulations to two past CBC Awardees, Jonathan Licht (NU, currently at the University of Florida Health Cancer Center) and Neil Kelleher, NU, on their recent publication in the Journal of Biological Chemistry, titled “Defining the NSD2 interactome: PARP1 PARylation reduces NSD2 histone methyltransferase activity and impedes chromatin binding.” Using proximity-based labelling (BioID) combined with label-free quantitative mass spectrometry the authors identify several candidate protein partners of NSD2 – a histone methyltransferase whose increased activity has been linked to blood cancers such as multiple myeloma (MM) and acute lymphoblastic leukemia. One of the identified partners in MM cells is a DNA damage regulator, Poly [ADP-ribose] polymerase 1 (PARP1), which appears to down-regulate NSD2 activity via a process called PARylation upon oxidative stress. The apparent crosstalk between PARylation and histone methylation and its role in DNA damage response, transcriptional regulation and other pathways is also discussed.
Senior and corresponding author on the paper, Jonathan D. Licht, MD, is the Director of the University of Florida Health Cancer Center, holding the Marshall E. Rinker, Sr. Foundation and David B. and Leighan R. Rinker Chair. Previously, Dr. Licht was Chief of Hematology/Oncology at NU and an Associate Director of the Robert H. Lurie Comprehensive Cancer Center. While at NU, Licht received a CBC Postdoctoral Research Award (2014) with Jon Oyer, a postdoc in his lab. In 2012 Licht was awarded a CBC Exploratory Workshop Award and the same year, he was an invited speaker at the 10th CBC Annual Symposium on “Epigenomics.” Neil Kelleher, a co-authors on the paper is a CBC Senior Investigator, who was hired in 2010 by NU with help of a generous CBC Recruitment Funds Award. Kelleher is Faculty Director, Northwestern Proteomics, and the Walter and Mary Elizabeth Glass Professor of Chemistry, Molecular Biosciences, Chemistry and Medicine at Feinberg School of Medicine. His other ties to the CBC are listed below.
Publication linked to CBC funding*:
Huang X, LeDuc RD, Fornelli L, Schunter AJ, Bennett RL, Kelleher NL, Licht JD. Defining the NSD2 interactome: PARP1 PARylation reduces NSD2 histone methyltransferase activity and impedes chromatin binding. J Biol Chem. 2019 Jun 27. [Epub ahead of print] (PubMed)
ABSTRACT
NSD2 is a histone methyltransferase that specifically dimethylates histone H3 lysine 36 (H3K36me2), a modification associated with gene activation. Dramatic overexpression of NSD2 in t(4;14) multiple myeloma (MM) and an activating mutation of NSD2 discovered in acute lymphoblastic leukemia (ALL) are significantly associated with altered gene activation, transcription and DNA damage repair. The partner proteins through which NSD2 may influence critical cellular processes remain poorly defined. In this study, we utilized proximity-based labelling (BioID) combined with label-free quantitative mass spectrometry to identify high confidence NSD2 interacting partners in MM cells. Represented in the top 24 proteins identified were involved in maintaining chromatin structure, transcriptional regulation, RNA pre-spliceosome assembly, and DNA damage. Among these, an important DNA damage regulator, Poly [ADP-ribose] polymerase 1 (PARP1), was discovered. PARP1 and NSD2 have been found to be recruited to DNA double strand breaks (DSBs) upon damage and H3K36me2 marks are enriched at damage sites. We demonstrate that PARP1 regulates NSD2 via PARylation upon oxidative stress. In vitro assays suggest the PARylation significantly reduces NSD2 histone methyltransferase activity. Furthermore, PARylation of NSD2 inhibits its ability to bind to nucleosomes and further get recruited at NSD2 regulated genes, suggesting PARP1 regulates NSD2 localization and H3K36me2 balance. This work provides clear evidence of crosstalk between PARylation and histone methylation and offers new directions to characterize NSD2 function in DNA damage response, transcriptional regulation and other pathways.
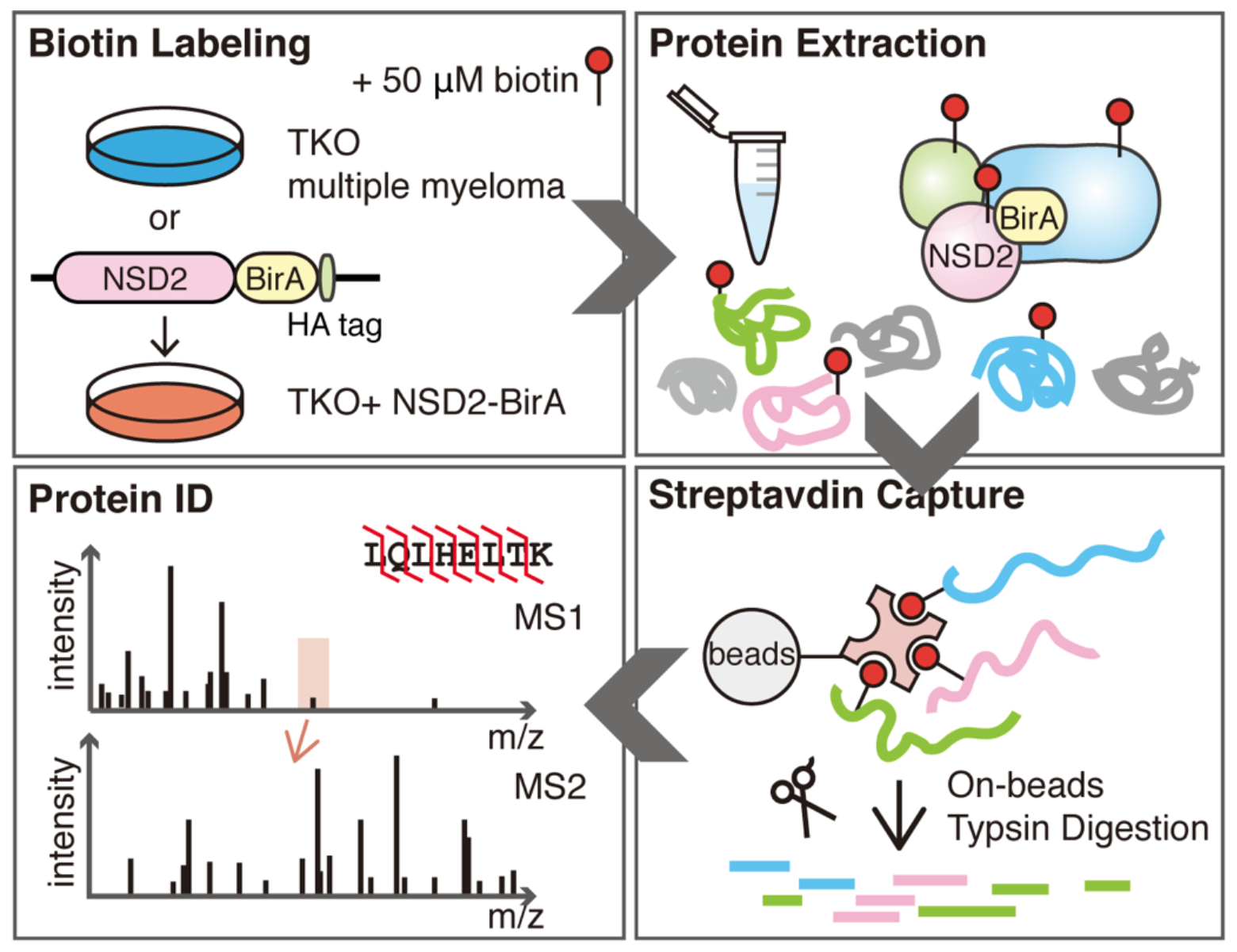
NSD2 interacting partners identified by BioID. Study design: TKO control cells or TKO MM cells stably expressing NSD2-BirA were cultured in media supplemented with 50 μM biotin for 48 hours, and then lysed. Biotinylated proteins were captured by streptavidin beads. On-bead trypsin digestion was performed, and resulting peptides were desalted and identified by nanocapillary LC-MS. (Source: www.jbc.org)
Featured CBC Community member(s):
Jonathan D. Licht, NU (currently at the University of Florida Health Cancer Center)
- CBC Postdoctoral Award (2014):
▸ A novel CRISPR application to discover interactions between genomic regulatory regions
PIs: Jon Oyer (postdoc) Jonathan D. Licht (NU) - CBC Exploratory Workshop (2012):
▸ Control of cellular differentiation and gene expression by 5- hydroxymethlcytosine (5-hmc)
Jonathan D. Licht (NU) — Organizer - 10th Annual CBC Symposium (2012):
▸ Epigenomics
Jonathan D. Licht (NU) — Symposium Speaker
Neil Kelleher, NU
- CBC Exploratory Workshop (2013):
▸ The CBC Exploratory Workshop on Cellular Heterogeneity
Neil Kelleher (NU) – Workshop Organizer and Speaker - CBC Science Day (2011):
Neil Kelleher (NU) – Science Day Speaker - CBC Catalyst Award (2010):
▸ Phosphoproteomic Analysis of NADPH Oxidase Activation
PIs: Neil Kelleher (NU) and Richard Ye (UIC) - *CBC Recruitment Resources Award (2010):
▸ CBC Senior Investigator
PI: Neil Kelleher (NU) - CBC Seminar (2010):
▸ Top Down Mass Spectrometry: Has Its Decade Now Come?
Neil Kelleher (NU, UIUC then) – Seminar Speaker
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
July 5, 2019
▸ New insight into liver cancer treatment
CBC Awardee Rick Silverman and a CBC Senior Investigator Neil Kelleher, NU, collaborate on developing therapeutics for hepatocellular carcinoma (HCC)
June 17, 2019
▸ Copper centers revealed with top-down mass spectrometry
Three CBC Awards contribute to today’s publication in Nature Communications!
May 13, 2019
▸ Battling epigenetic lymphomas
CBC Senior Investigator Neil Kelleher, NU, contributes to a new publication identifying a potential novel therapeutic pathway to treat the so called EZH2 dysregulated lymphomas
May 7, 2019
▸ Proteoforms explained
A recent review in Proteomics, co-authored by a CBC Senior Investigator and proteomics expert, Neil Kelleher, NU
August 9, 2018
▸ Top-down Proteomics
CBC Senior Investigator, Neil Kelleher, NU, explains the advantages of “top-down” versus “bottom-up” proteomics in early cancer detection and progression
November 13, 2017
▸ New insights into regulation of gene expression: work from the Shilatifard lab (NU) with contributions from two CBC scientists, Neil Kelleher and Jeffrey Savas.
November 7, 2017
▸ Highlighting progress in cancer research supported in part through a CBC Lever Award. Spotlight on Vadim Backman, Tom O’Halloran and Andrew Mazar.
October 5, 2017
▸ CBC Senior Investigator, Neil Kelleher, NU, deciphers molecular assembly of a gut toxin, colibactin
February 9, 2016
▸ “Clasping” Collaboration
Three CBC Scientists Join Forces to Develop Exceptional-Quality Antibodies Displaying an Unprecedented Mode of Action

