June 5, 2019 | Jola Glotzer
Meet a double-agent, MnSOD
David Gius, NU, explains in a recent Nature Communications paper — partially funded by a CBC Catalyst Award — how MnSOD can act as a pro-, or an anti-cancer agent

David Gius, MD, PhD, professor in Radiation Oncology and Pharmacology, was the senior author of the study published in Nature Communications.
Congratulations to David Gius, NU, on his recent publication in Nature Communications, titled “Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter.” The paper addresses an interesting phenomenon observed in the case of manganese superoxide dismutase (MnSOD), which is a known tumor suppressor, but can also play a role of a tumor promoter in situations where cancer has already developed. Turns out, a single amino-acid modification—lysine 68 acetylation—causes the protein to form homo-tetrameric complexes which have detoxification properties. Once, however, acetylation is disrupted in pathological stress conditions, such as cancer, the monomeric MnSOD gains tumor promoter properties. The authors entertain a hypothesis that perhaps in vivo an equilibrium of monomeric and tetrameric MnSOD exists, which, depending on the level of metabolic stress the cell is experiencing, can be tipped in any direction. In consequence, one of the dual modes of action of MnSOD prevails, protecting or further hurting the cell.
A CBC Catalyst Award that Gius received in 2015 for the project “Fluorescent Probes for Live-cell Imaging of Sirtuin Activity: Application to Breast Cancer” partially funded the publication. Gius is senior author on the current study. Another CBC awardee, Karla Satchell, is one of the co-authors on the Nature Communications paper.
Publication attributed to CBC funding*:
Zhu Y, Zou X, Dean AE, Brien JO, Gao Y, Tran EL, Park SH, Liu G, Kieffer MB, Jiang H, Stauffer ME, Hart R, Quan S, Satchell KJF, Horikoshi N, Bonini M, Gius D. Lysine 68 acetylation directs MnSOD as a tetrameric detoxification complex versus a monomeric tumor promoter. Nat Commun. 2019 Jun 3;10(1):2399. (PubMed)
ABSTRACT
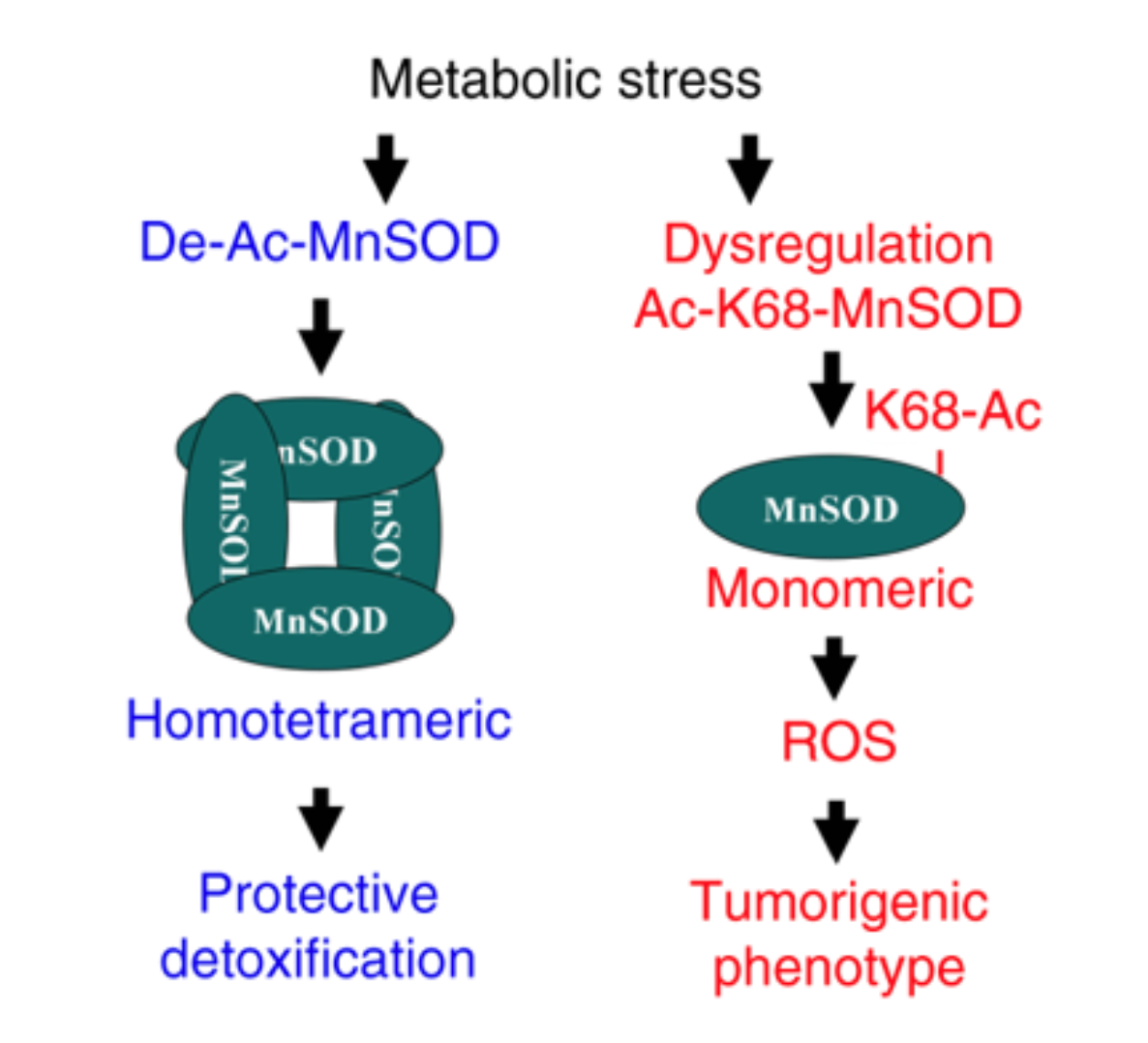
Schematic of the dichotomous role for MnSOD in normal cells (i.e., protection, blue) versus tumor promoter and/or and Tam resistance (red). (Source: Nature Communications)
Manganese superoxide dismutase (MnSOD) functions as a tumor suppressor; however, once tumorigenesis occurs, clinical data suggest MnSOD levels correlate with more aggressive human tumors, implying a potential dual function of MnSOD in the regulation of metabolism. Here we show, using in vitro transformation and xenograft growth assays that the MnSOD-K68 acetylation (Ac) mimic mutant (MnSODK68Q) functions as a tumor promoter. Interestingly, in various breast cancer and primary cell types the expression of MnSODK68Q is accompanied with a change of MnSOD’s stoichiometry from a known homotetramer complex to a monomeric form. Biochemical experiments using the MnSOD-K68Q Ac-mimic, or physically K68-Ac (MnSOD-K68-Ac), suggest that these monomers function as a peroxidase, distinct from the established MnSOD superoxide dismutase activity. MnSODK68Q expressing cells exhibit resistance to tamoxifen (Tam) and cells selected for Tam resistance exhibited increased K68-Ac and monomeric MnSOD. These results suggest a MnSOD-K68-Ac metabolic pathway for Tam resistance, carcinogenesis and tumor progression.
ACKNOWLEDGMENTS
D.G. is supported by 2R01CA152601-A1, 1R01CA152799-01A1, 1R01CA168292-01A1, 1R01CA214025-01, the Avon Breast Cancer Foundation, the Lynn Sage Cancer Research Foundation, the Zell Family Foundation, and the Chicago Biomedical Consortium, as well the Searle Funds at The Chicago Community Trust. M.B. is supported by a U.S. Department of Defense grant 67263-RT-REP and an NIH 1RO1HL125356 award. Y.Z. is supported by a Robert H. Lurie Comprehensive Cancer Center Translation Bridge Fellowship Award. Y.G. was supported in part by the Chicago Cancer Baseball Charities at the Lurie Cancer Center of Northwestern University. K.J.F.S. is supported by 5R01AI092825-07. Imaging work was performed at the Northwestern University Center for Advanced Microscopy, which is generously supported by NCI CCSG P30 CA060553, awarded to the Robert H. Lurie Comprehensive Cancer Center. Proteomics services were performed by the Northwestern Proteomics Core Facility, generously supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569. The structural analyses of MnSOD were performed at the Northwestern University Structural Biology Facility, which is generously supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center. The bacterial MnSOD expression and lysine acetylation tRNA mutant plasmids were a gift from Jiangyun Wang, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China.
See also:
▸ Uncovering Enzyme’s Dual Role in Cancer Suppression and Growth, by Anna Williams, for NU Medicine News Center, published on June 3, 2019
Featured CBC Community member(s):
David Gius, NU
- *CBC Catalyst Award (2015):
▸ Fluorescent Probes for Live-cell Imaging of Sirtuin Activity: Application to Breast Cancer
PIs: David Gius (NU) and Bryan Dickinson (UChicago)
Karla Fullner Satchell, NU
- CBC Postdoctoral Research Award (2015):
▸ Identifying Inhibitors of Novel Ras Bacterial Protease by High Throughput Screening
PIs: Marco Biancucci (postdoc) and Karla Fullner Satchell (NU)
ARTICLES PUBLISHED IN THE PAST ABOUT THE FEATURED CBC COMMUNITY MEMBER(S):
February 5, 2019
▸ Macrophages – a dance of life with death
Five (!) CBC community members collaborate on how to speed up heart recovery after an infarct
August 31, 2018
▸ Desmoplakin and heart failure
Two NU researchers with ties to CBC, Kathleen Green and Karla Satchell, provide new insight into the molecular mechanisms behind the disease
