November 29, 2017
Cytoplasmic component of COMPASS identified as a promising target for cancer therapeutics development—a new finding supported in part through CBC funding!
A new study, recently published in the journal Genes and Development, titled “A cytoplasmic COMPASS is necessary for cell survival and triple-negative breast cancer pathogenesis by regulating metabolism,” pinpoints a novel, cytoplasmic function for a member of the COMPASS family, that may be a potential target for drug development in human diseases, including cancer. Two Northwestern University Feinberg School of Medicine professors, Ali Shilatifard and Jeffrey Savas, are senior co-authors on the publication. CBC funding to Savas—he is a recipient of a CBC Catalyst Award (2015)—has contributed to the published work. Congratulations!
New Insights Into Protein Reveal Potential Therapy for Breast Cancer
Northwestern Medicine News Center | By ANNA WILLIAMS | November 27, 2017
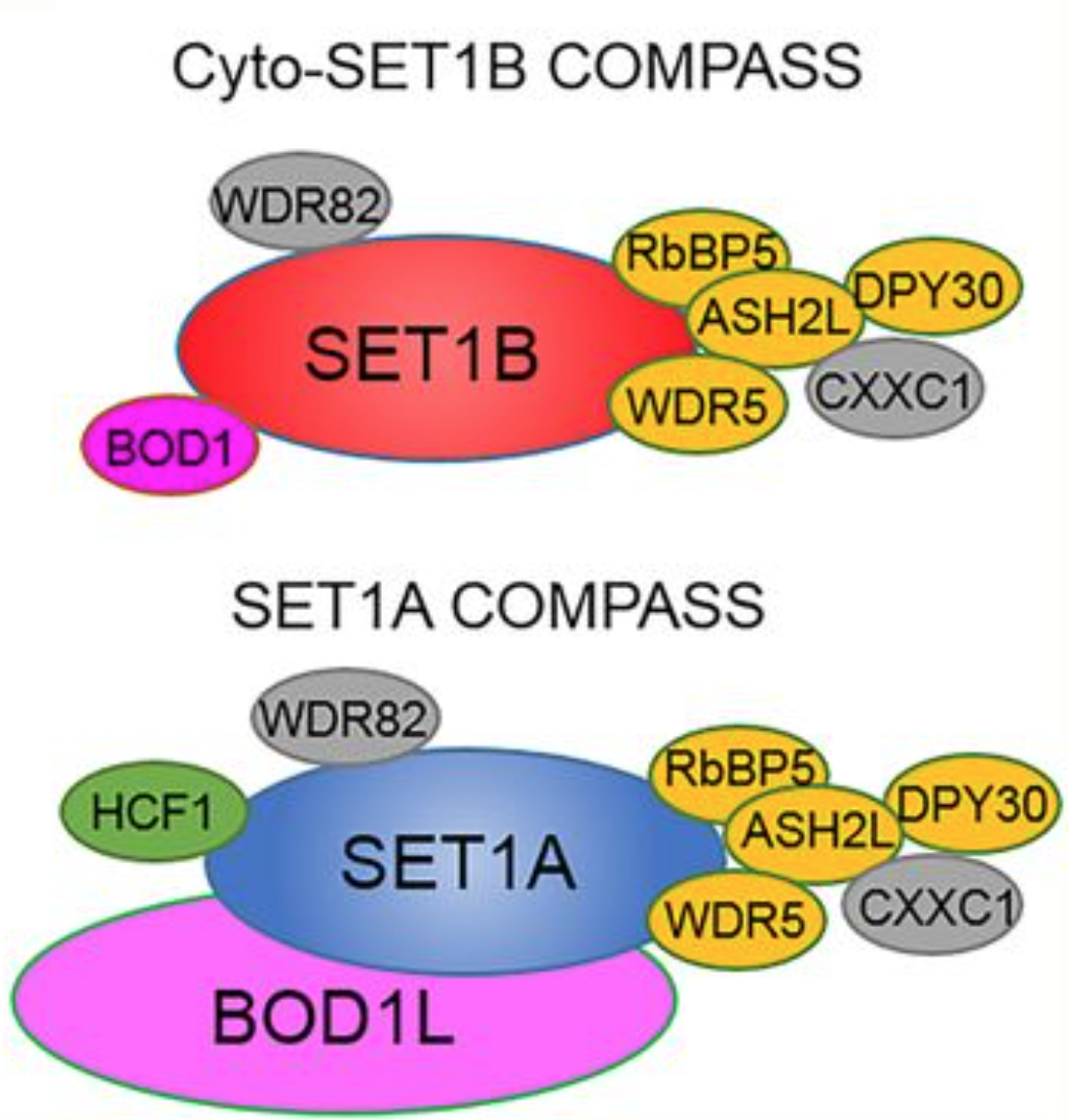
Cartoon depictions of cyto-SET1B COMPASS and SET1A COMPASS. (Orange) WARD complex, which contains WDR5, ASH2L, RBBP5, and DPY30; (gray) SET1A/B COMPASS complex-specific subunits WDR82 and CXXC1; (pink) cyto-SET1B COMPASS-specific subunit BOD1. Credit: Genes and Development.
Northwestern Medicine scientists have discovered a new function for a protein called SET1B in the cytoplasm of cells, and demonstrated that targeting its role in regulating cellular metabolism may be able to treat triple-negative breast cancer.
The findings were published in the journal Genes and Development.
“This is a major discovery,” said principal investigator Ali Shilatifard, PhD, the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics.
Lu Wang, PhD, a postdoctoral fellow in Shilatifard’s laboratory, was the first author of the study.
SET1B is one of the six members of COMPASS, a family of enzymes which was first characterized by Shilatifard close to 20 years ago. The complex is known to be critical to gene expression: COMPASS catalyzes a key molecular event called histone methylation, which influences whether genes are turned on or off.
Dysregulation and mutations in some COMPASS genes have since been implicated in many types of human diseases, including cancer. But the function of SET1B, and its relationship with cancer, had remained unclear.
In the current study, the scientists first discovered that the majority of SET1B resides in the cytoplasm of cells — a surprising finding, given that all other members of COMPASS are found mainly in the nucleus.
“SET1B is also essential to the viability of different cancer cells, especially human breast cancer,” Wang explained. “When the gene is deleted in triple-negative breast cancer using the gene-editing tool CRISPR, the cells do not survive. Interestingly, normal epithelia cells are fine with the depletion of SET1B.”
To further understand SET1B’s link to cancer growth, the scientists demonstrated that loss of SET1B leads to increased expression of several genes that modulate fatty acid metabolism — indicating a novel function for SET1B in regulating metabolic processes.
The Shilatifard laboratory also explored how these findings might offer novel strategies for treating breast cancer.
“At first, we thought about how to target SET1B — but a crystal structure of this 300kd protein doesn’t exist, so we can’t design a small molecule targeting it,” Wang explained. “So we looked at the major downstream genes to target instead.”

Ali Shilatifard, PhD, NU
ADIPOR1 is one of the genes the scientists discovered was regulated by SET1B. ADIPOR1 is the receptor for adiponectin, a hormone that is known to have anti-diabetic effects. A Japanese research group had already developed a small molecule agonist drug to activate that receptor, called AdipoRon. As reported in Nature in 2013, AdipoRon improved insulin resistance and extend the lifespan of obese diabetic mice.
Given their discovery about the close relationship between SET1B and ADIPOR1, the Northwestern scientists decided to investigate using AdipoRon to treat triple-negative breast cancer. Triple-negative breast cancer is a type of breast cancer that lacks the three receptors targeted in common therapies, and as such, can be difficult to treat.
The team discovered that AdipoRon was capable of killing triple-negative breast cancer cells in vitro, and further demonstrated in a mouse model of the cancer that treatment with the drug significantly reduced tumor size and increased animal survival.
Wang noted that the current study is the first to demonstrate how this small molecule drug, which has been used for the treatment of diabetes, could be used to treat human cancer.
In future studies, Shilatifard and his team intend to further analyze clinical data that suggest a correlation between SET1B gene expression and breast cancer patient survival, as well as investigate the development of other compounds that might target SET1B for cancer treatments.
Shilatifard is also a professor of Pediatrics and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Along with other members of the Shilatifard lab, the paper was also co-authored by Rintaro Hashizume, MD, PhD, assistant professor of Neurological Surgery and of Biochemistry and Molecular Genetics, and Jeffrey Savas, PhD, assistant professor of Neurology in the Division of Movement Disorders, Medicine and Pharmacology.
The study was partially supported by Northwestern University Feinberg School of Medicine, R00 DC-013805 and the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust and the Training Program in Signal Transduction and Cancer T32 CA070085-19. The COMPASS studies in Shilatifard’s laboratory are supported by a generous grant from the Mary Kay Foundation and an Outstanding Investigator Award grant from the National Cancer Institute R35CA197569.
Source:
Adapted (with modifications) from Northwestern Medicine News Center, posted by Anna Williams on November 27, 2017.
Publication attributed to the CBC-funding:
Wang L, Collings CK, Zhao Z, Cozzolino KA, Ma Q, Liang K, Marshall SA, Sze CC, Hashizume R, Savas JN, Shilatifard A. A cytoplasmic COMPASS is necessary for cell survival and triple-negative breast cancer pathogenesis by regulating metabolism. Genes Dev. 2017 Oct 15;31(20):2056-2066. (PubMed)
See also:
CBC Awards:
CBC Catalyst Award (2015):
PIs: Christopher Gomez (UChicago) and Jeffrey Savas (NU) for the project:
▸ Overlapping cistrons within mammalian mRNAs

